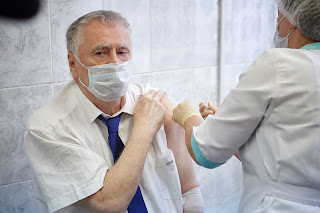
Octuple-COVID-19-vaccinated politician dies from COVID-19 at 75
On September 2 of 2020, Vladimir Zhirinovsky, the leader of the Liberal Democratic Party (LDPR) of Russia, received his first dose of COVID-19 vaccine - as a participant of Gam-COVID-Vac (Sputnik V) clinical trials. 3 weeks later he got the second dose with no side effects.
Sputnik V (Спутник V) is an adenovirus viral vector vaccine for COVID-19 developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia. It is the world's first registered combination vector vaccine for the prevention of COVID-19, having been registered on 11 August 2020 by the Russian Ministry of Health. Gam-COVID-Vac was initially approved for distribution in Russia and then in at least 59 other countries on the preliminary results of Phase I–II studies eventually published on 4 September 2020. Both doses of Sputnik V contain non-replicating viral vectors. The first component uses adenovirus type 26 (AD26), and the second — which a person receives 21 days later — contains adenovirus type 5 (AD5).
Several of the presently approved vaccines against SARS-Coronavirus-2 (AstraZeneca/Oxford University, Johnson & Johnson's Janssen COVID-19 Vaccine, and Sputnik V) are based on adenovirus DNA vectors as carriers for the genetic information for the SARS-COV-2 spike glycoprotein.Since antibody titers decline 3-month post-vaccination, In December 2020, Zhirinovsky received Sputnik Light, essentially the 3rd booster dose of the Sputnik V vaccine. The Sputnik Light vaccine is made from Ad26, which is the first part of the Sputnik V vaccine. Sputnik Light is authorized for use in Russia as a single-dose vaccine. Johnson and Johnson’s single-dose vaccine is also based on human adenovirus serotype number 26 (rAd26)
REFERENCES
Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Shcheblyakov DV, Dzharullaeva AS, Grousova DM, Erokhova AS, Kovyrshina AV, Botikov AG, Izhaeva FM. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. The Lancet. 2020 Sep 26;396(10255):887-97.
Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, Botikov AG. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet. 2021 Feb 20;397(10275):671-81.
Kozlovskaya LI, Piniaeva AN, Ignatyev GM, Gordeychuk IV, Volok VP, Rogova YV, Shishova AA, Kovpak AA, Ivin YY, Antonova LP, Mefyod KM. Long-term humoral immunogenicity, safety and protective efficacy of inactivated vaccine against COVID-19 (CoviVac) in preclinical studies. Emerging microbes & infections. 2021 Jan 1;10(1):1790-806.
Kozlovskaya L, Piniaeva A, Ignatyev G, Selivanov A, Shishova A, Kovpak A, Gordeychuk I, Ivin Y, Berestovskaya A, Prokhortchouk E, Protsenko D. Isolation and phylogenetic analysis of SARS-CoV-2 variants collected in Russia during the COVID-19 outbreak. International Journal of Infectious Diseases. 2020 Oct 1;99:40-6.
Жириновский, Владимир Вольфович — Википедия (wikipedia.org)
Что стало причиной смерти Владимира Жириновского? | В России | Политика | Аргументы и Факты (aif.ru)
Умер Владимир Жириновский // НТВ.Ru (ntv.ru)


Comments
Post a Comment