Long Breakthrough COVID: 4 case reports
A previously healthy 47 year-old woman was evaluated at our post-COVID clinic for 7 months of PASC symptoms. She developed acute COVID-19 infection in the summer of 2021 and had received two doses of the BNT162b2 (Pfizer-BioNTech) vaccine 6 months prior to the onset of her infection. Her acute symptoms included cough, sore throat, altered smell and taste, headache, fever, chills, body aches, chest pressure, and fatigue, which were managed with home care. COVID-19 infection was confirmed by PCR test. Most acute symptoms resolved after 48 hours, but over the next several months she continued to suffer severe fatigue, cognitive difficulties, post-exertional malaise, insomnia, tachycardia, chest pressure, and body aches resulting in significant functional debilitation and a leave of absence from work. She also experienced headaches and hair loss, both of which self-resolved.
6-months post-infection, the patient was potentially exposed to COVID-19 again and developed new symptoms of headache, congestion, sore throat, low grade fever, sweats, and malaise. She tested negative for COVID-19 by antigen test but was started on a 5-day course of nirmatrelvir (300mg)-ritonavir (100mg) twice daily on day 3 of her acute symptoms by her primary care provider, given considerations of a potential false negative and her prior breakthrough infection history. Her acute flu-like symptoms had already begun to self-improve by day 3, but she noticed rapid improvement of her pre-existing PASC symptoms after taking the antivirals. At 7-months post-initial Page 3/5 infection, her PASC symptoms had resolved, and she reported being back to her normal, pre-COVID health status and function including working fulltime and exercising rigorously.
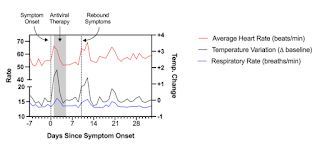
A 48-year-old man with a past medical history of presumed Bechet’s disease on colchicine developed fever, worsening headache, and pharyngitis in Spring 2022. He had previously received 2 doses of the Moderna SARS-CoV-2 vaccine and 1 dose of the Pfizer-BioNTech vaccine, most recently 5 months prior. A rapid antigen test was positive, as was a confirmatory PCR test. He was prescribed a 5-day course of nirmatrelvir/ritonavir, which he initiated within 24 hours of symptom onset, and experienced rapid improvement in his systemic symptoms. However, approximately four days following completion of the 5-day course, he experienced rebound symptoms with recurrence of fever, fatigue, rhinorrhea, cough, chest pain, rash on his upper and lower extremities, and trouble concentrating (“brain fog”). During this period, he wore a personal fitness device which recorded certain physiologic measurements including heart rate, respiratory rate, and change from baseline body temperature (see Fig.). Approximately 3 weeks following the positive test and despite prior antiviral therapy, he experienced worsening of his fatigue and associated chest soreness, palpitations, brain fog, and symptoms of post-exertional malaise, which have now persisted beyond 30 days following initial symptom onset.
A 42-year-old man with no significant medical history developed rhinorrhea and pharyngitis in early 2022, followed by fatigue, myalgia, and a pruritic rash on his upper extremities and groin that persisted for approximately 10 days. He had previously received 3 doses of the Pfizer-BioNTech SARS-CoV-2 vaccine, most recently 2 months prior. A SARS-CoV-2 antigen test was positive when performed two days after symptom onset; multiple additional antigen tests were subsequently positive. On Day 11, an antigen test was negative and all of his symptoms resolved. Approximately two weeks later, he experienced new onset of severe myalgia and bone pain across his upper body, which he described as post-exertional soreness in the absence of exertion. There was an associated increase in fatigue, as well as heightened awareness of breathing (described as “lung soreness”). These symptoms persisted, and he contacted his primary care doctor for evaluation approximately 10 days later. At this visit, he noted that he was experiencing ongoing fatigue, and described being at 80% of his pre-COVID baseline health. Laboratory testing was notable only for vitamin D insufficiency (25 ng/mL); a chest X-ray showed no abnormalities. Approximately 7 weeks following initial symptom onset, his symptoms worsened. He experienced ongoing myalgia, severe fatigue, post-exertional malaise, and trouble with concentration (“brain fog”). These symptoms profoundly impacted his ability to perform his activities of daily living, and he felt substantially debilitated, reporting that he was at 40–50% of his pre-COVID baseline health and that he was spending the majority of the day resting. He began to seek care for Long COVID because he was concerned about the duration for which his symptoms had persisted. As his symptoms continued to worsen, he was re-exposed to SARS-CoV-2 when his spouse and children tested positive on antigen tests. A repeat antigen test was negative, but he experienced further worsening of his symptoms which his provider attributed to possible re-infection with SARS-CoV-2. In this context, he received a prescription for nirmatrelvir/ritonavir. Within days of re-exposure, the patient began to note an improvement in his persistent symptoms, while his family members continued to experience worsening symptoms. After symptomatic improvement for 1–2 days, he initiated a 5-day course of nirmatrelvir/ritonavir. During this period, he continued to experience improvement in his symptoms. While they have not resolved entirely, he reports that he is gradually approaching his baseline health.
A 43-year-old woman with no significant medical history developed cough and pharyngitis in Spring 2022. She had previously received 3 doses of the Pfizer-BioNTech SARS-CoV-2 vaccine, most recently 4 months prior. While a PCR test was initially negative, she and one of her children subsequently tested positive on an antigen test 5 days later. She did not initially receive antiviral therapy. Over the course of the subsequent 3 weeks, she began to experience worsening fatigue and malaise, with associated myalgia and trouble concentrating (“brain fog”); 3 weeks following initial symptom onset she was spending the majority of the day resting and was unable to easily complete her activities of daily living. She received a prescription for nirmatrelvir/ritonavir, which she began 25 days following initial symptom.
REFERENCES
Peluso MJ, Anglin K, Durstenfeld MS, Martin JN, Kelly JD, Hsue PY, Henrich TJ, Deeks SG. Effect of oral nirmatrelvir on Long COVID symptoms: a case series. DOI: https://doi.org/10.21203/rs.3.rs-1617822/v2
Geng LN, Bonilla HF, Shafer RW, Miglis MG, Yang PC. Case Report of Breakthrough Long COVID and the Use of Nirmatrelvir-Ritonavir. DOI: https://doi.org/10.21203/rs.3.rs-1443341/v1

Comments
Post a Comment