Hepatitis triggered by a complex interplay with SARS-CoV-2 vaccination
Two more cases of hepatitis/colitis and hepatitis/MIS-C to add to the January post.
New report describes a case similar to previously published case reports of hepatitis after COVID-19 vaccination.
This article reports a case of hepatitis and colitis in a 52-year-old woman who was undergoing immunotherapy and was HBV positive 10 days after receiving the first Pfizer-BioNTech COVID-19 vaccine dose. Because both ICIs and the COVID-19 vaccines stimulate the immune response, the authors hypothesized that these vaccines may increase the incidence of irAEs during ICI treatment. There is a complex interplay between the immune-mediated reaction triggered by the vaccination and PD-L1 co-administration.
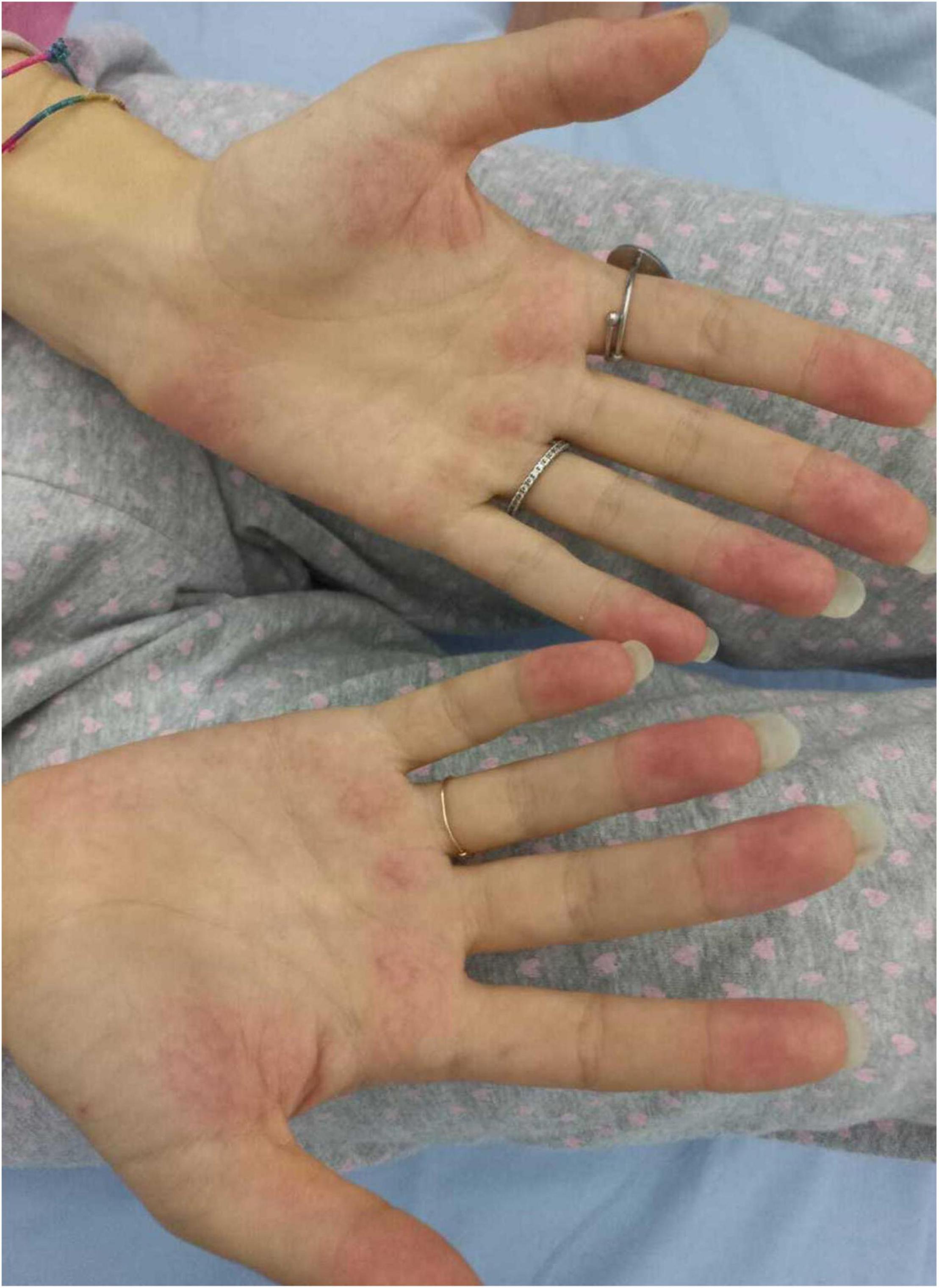
Another case describes a 17-year-old female hospitalized for a history of fever, asthenia, cough, anorexia, abdominal pain, and vomiting. The patient has a positive familial history for autoimmune disorders (inflammatory bowel diseases, connective tissue diseases, and multiple sclerosis). She had paucisymptomatic SARS-CoV-2 infection approximately 1 year before this episode and was vaccinated with two doses of anti-SARS-CoV-2 mRNA vaccine (Comirnaty), 5 and 4 months before hospitalization, respectively, with a detectable immune response. She had a high-risk contact (more than 6 h, without protective face mask) with a SARS-CoV-2 positive patients 10 days before hospitalization. On the basis of the clinical picture of multi-systemic involvement (fever, hepato-splenomegaly, hypoalbuminemia, pulmonary nodular involvement, conjunctivitis, erythematous rash, acral vasculitis, and raised inflammatory markers) and the history of recent exposure to SARS-CoV-2, the diagnosis of MIS-C was posed. A week after the introduction of treatment, she presented a relapse of the abdominal pain, associated with elevation of amylase and lipase, and the patient underwent a chest and abdomen CT, that showed a marked improvement of the pulmonary radiologic findings and features consistent with hepatitis and pancreatitis. In the following days, she continued the corticosteroid therapy and received parenteral nutrition for 2 days, resulting in a progressive resolution of the clinical and laboratory picture.
Unusual epidemic of hepatitis in children has been widely reported in recent weeks. So far, it affected at least 176 children in the UK and more than 500 worldwide. It might be caused by an interplay with AAV-2, another adenovirus AdV-F41 and/or Human herpes virus-6, but no one has a definite answer to its causes yet.
REFERENCES
Lasagna A, Lenti MV, Cassaniti I, Sacchi P. Development of hepatitis triggered by SARS-CoV-2 vaccination in patient with cancer during immunotherapy: a case report. Immunotherapy. 2022 Jun 13. doi: 10.2217/imt-2021-0342. Epub ahead of print. PMID: 35694999.
Consolini R, Costagliola G, Spada E, Colombatto P, Orsini A, Bonuccelli A, Brunetto MR, Peroni DG. Case Report: MIS-C With Prominent Hepatic and Pancreatic Involvement in a Vaccinated Adolescent–A Critical Reasoning. Frontiers in Pediatrics. 2022;10.
Camacho-Domínguez L, Rodríguez Y, Polo F, Gutierrez JC, Zapata E, Rojas M, Anaya JM. COVID-19 vaccine and autoimmunity. A new case of autoimmune hepatitis and review of the literature. Journal of translational autoimmunity. 2022 Jan 4:100140.
Rudan I. The COVID-19 pandemic: SARS-CoV-2, childhood hepatitis and monkeypox raise five new questions for the global health research community. Journal of Global Health. 2022;22.
Binnicker M. Adenovirus or Covid-19: What is behind the outbreak in child hepatitis cases? Available from: https://www.forbes.com/sites/coronavirusfrontlines/2022/05/19/adenovirus-or-covid-19-what-is-behind-the-outbreak-in-child-hepatitis-cases. Accessed: June 15th 2022.


Comments
Post a Comment