ANCA-associated vasculitis
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare disease with an annual incidence of approximately 20 per million population in Europe and North America. The aetiopathogenesis is complex and is mainly driven by the gene environment interactions. Genes associated with this condition include HLA-DQ (variations in which have been linked to asymptomatic COVID), IL-5 and GPA33. COVID-19 heralds a poor prognosis in those with preexisting AAV with over 50% patients having severe disease. Loss of smell is observed in AAV population more frequently (~50%) than in others (~35%).
70-year-old Chinese woman presented with poor appetite and nausea 4 hours after receiving the first dose of CoronaVac vaccine. These symptoms progressively worsened, followed by fatigue and foamy urine 10 days later. Her medical history included hypertension, hyperlipidemia, and kidney stones. She had penicillin allergy and no family history of ANCA-associated vasculitis. Treatment helped to improve renal function.
A 59-year-old male with a past medical history of hypertension and ischemic heart disease presented with fever, malaise, and polyarthralgia associated with nausea and anorexia that started days after his second dose of the Pfizer COVID-19 vaccine. He had no history of smoking, substance abuse, COVID-19 infection and no family history of autoimmune disease. Serological testing showed positive ANCA titers (>131 IU/ml).
A 58 years-old female presented with fatigue, paleness, arthralgia on hands, knees, ankles, foamy urine, and elevated blood pressure 5 days after Oxford-AstraZeneca COVID-19 vaccination. Blood tests showed serum creatinine of 2.2 mg/dL (baseline creatinine of 1.0 mg/dL). Additional tests revealed hematuria, hypercholesterolemia, and severe anemia.
A 51-year-old previously healthy male presented with a 3-day history of low-grade fever with debilitating inflammatory polyarthritis, 15 days after ChAdOx1 nCoV-19 Vaccine (COVISHIELD- manufactured by Serum Institute of India Pvt Ltd.). He was diagnosed with PR3-AAV and had resolution of arthralgias 20 weeks later, after treatment.
A 72-year-old female with no specific past medical history presented with tinnitus, nasal congestion, and bloody rhinorrhea. Her constitutional symptoms developed after she received the first dose of the ChAdOx1 nCoV-19 (Oxford AstraZeneca) COVID-19 vaccine, and her symptoms were aggravated after she received the second dose of the ChAdOx1 nCoV-19 vaccine. She underwent tympanostomy tube insertion and received antibiotic therapy for 2 weeks. However, after she received her third (booster) dose, the mRNA1273 (Moderna) vaccine 4 months later, she experienced anorexia, abdominal pain, and febrile sensation. Her endoscopic findings revealed erosive gastritis. Serological tests revealed positive ANCA titers (> 120 IU/mL) and antibodies against MPO. Two months later, her constitutional symptoms improved after treatment.
A 75‐year‐old man with historical renal‐limited microscopic polyangiitis (MPA) AAV, previously in remission, presented with 3 days of haemoptysis, onset 5 weeks following the first dose of AstraZeneca vaccine. Kidney biopsy demonstrated AAV relapse, requiring ongoing kidney replacement therapy.
A 74‐year‐old man with MPA AAV, diagnosed 18 months previously with no prior kidney involvement, was referred with worsening kidney impairment, first noted 2 weeks following first AstraZeneca vaccine doze. His condition worsened after the second doze,but improved with treatment.
A 29-year-old healthy man developed purpuric skin lesions one week after his second AstraZeneca/Oxford COVID-19 vaccine which complicated by glomerulonephritis and gastrointestinal involvement. Vaccine-related IgA vasculitis was the most likely explanation.
REFERENCES
Ma Y, Huang T, Xu G. ANCA-associated vasculitis following the CoronaVac vaccination. Ther Adv Chronic Dis. 2022 Nov 14;13:20406223221125708. doi: 10.1177/20406223221125708. PMID: 36407020; PMCID: PMC9666871.
Prabhahar A, Naidu GS, Chauhan P, Sekar A, Sharma A, Sharma A, Kumar A, Nada R, Rathi M, Kohli HS, Ramachandran R. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: case-based review. Rheumatology international. 2022 Feb 5:1-0.
Baier E, Olgemöller U, Biggemann L, Buck C, Tampe B. Dual-Positive MPO-and PR3-ANCA-Associated Vasculitis Following SARS-CoV-2 mRNA Booster Vaccination: A Case Report and Systematic Review. Vaccines. 2022 Apr 21;10(5):653.
Uddin K, Mohamed K, Agboola A, Naqvi W, et al.: Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Renal Vasculitis Following COVID-19 Vaccination: A Case Report and Literature Review. Cureus. 2022.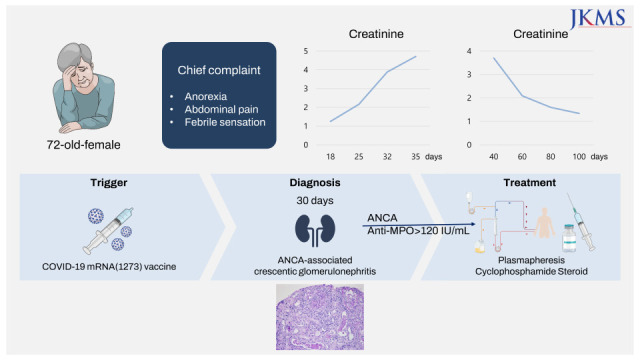


Comments
Post a Comment