STAT3-mediated adverse events following immunization with an mRNA vaccine
Genetic factors that increase the risk of COVID-19 vaccine-induced adverse events are currently being investigated. A new article is presenting a case of T-LGL, T-cell large granular lymphoma, a rare lymphoproliferative disorder, in a patient who received a COVID-19 mRNA vaccine. The authors analyzed the results of gene sequencing for pathogenic mutations but did not find any known variations associated with T-LGL, particularly not in STAT3 or STAT5b, harboring the most common gain-of-function mutations. However, the article provides evidence that the vaccine induces STAT3 activation through TLR stimulation, which may potentially exacerbate STAT3-dependent diseases. Interestingly, expansion of T cells causing progressive lymphadenopathy following mRNA vaccination has been reported in a patient with angioimmunoblastic T Cell lymphoma, indicating that further investigation is needed to understand the potential genetic and immune-related factors that increase the risk of lymphoma after vaccination.
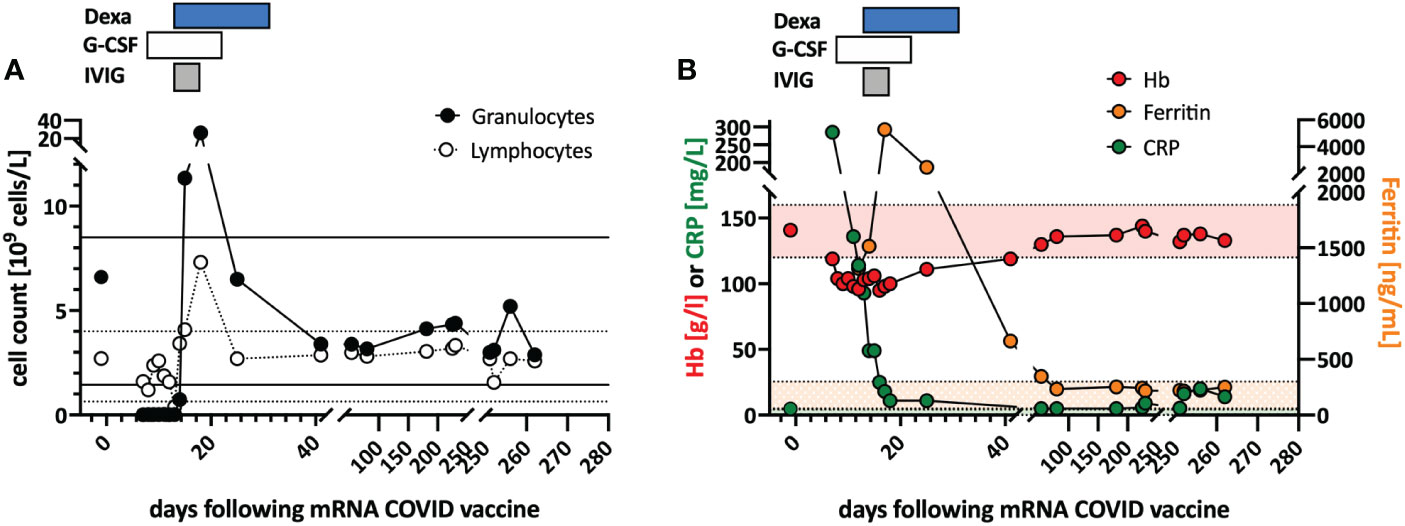
Previously healthy woman in her 60s had fatigue and malaise, starting 24 hours following the first dose of the COVID-19 vaccine mRNA-1273. On the sixth day post-vaccination, she developed fever (39°C), sore throat, coughing and vomiting. She denied taking non-steroidal anti-inflammatory drugs that could cause neutropenia (i.e. NSAIDs or metamizole). The physical examination was unremarkable and revealed no palpable lymph nodes or splenomegaly and no petechia, rash, or pharyngitis. The CT scans showed several marginally increased cervical lymph nodes without organomegaly, especially without splenomegaly. Systemic inflammation markers were very high with a C-reactive protein (CRP) of 285 mg/l (reference <5mg/l) and a ferritin peak level of 5276 ng/ml (reference <300ng/mL), compatible with macrophage activation (see Figure).
Tests for SARS-CoV2 and other infections (blood cultures, multiplexed PCR panels from stool and nasal swabs, serology for hepatitis viruses, CMV, and Parvovirus B19) were negative. PET-CT revealed pronounced diffuse hypermetabolism of the hematopoietic bone marrow and spleen. Bone marrow biopsy showed infiltration of CD8+ T cells and hypercellularity with severely reduced granulopoiesis, consistent with pure white cell aplasia.
A diagnosis of possible T-LGL leukemia with pure white cell aplasia was made.
Interstitial T-cell lymphocytosis with some linearity and intravascular spread of the T cells was suggestive of T-LGL. Immunophenotyping of the lymphocytes in the peripheral blood and bone marrow identified a large CD8+ T cell population (~45% of all T cells) expressing CD5dim, CD7, CD16partial, TCRαβ, HLA-DR and that was negative for CD56, TCRγδ, CD30, CD10, CD25, and PD1 in the clinical routine lab, compatible with T-LGL. The T- and B-cell clonality and translocation analysis showed a dominant TCRγ-rearrangement with a base pair length of 200 bp (type V-I) on a polyclonal background. There was no evidence of B-cell clonality or translocations (t(11;14), t(14;18)). Next-generation sequencing applying a custom lymphoma panel covering 68 genes commonly mutated in lymphomas (21, 22) identified no mutation, particularly not in STAT3 or STAT5b.
The incidence of neutropenia following immunization with the mRNA vaccine BNT162b2 was reported as 2.6 per 100’000 vaccinated subjects. Notably, none of these cases was severe (defined as <500/ul neutrophils) and the incidence of neutropenia is about 40-times higher in patients with COVID-19 (88.4 per 100’000 cases) (25). In a cohort of 342 patients with inborn errors of immunity (IEI), one patient with common variable immunodeficiency developed severe neutropenia three days following BNT162b2 immunization. The patient required treatment with granulocyte stimulating factor and systemic corticosteroid therapy. Interestingly, the patient was subsequently diagnosed with T-LGL (26). To our knowledge, there are no other reports of severe neutropenia in T-LGL. Recently, good COVID-vaccine immunogenicity was demonstrated in a small cohort of mostly treated T-LGL patients (27). Safety data were not reported in this study.
The authors hypothesized that mRNA-vaccine-mediated STAT3 activation stimulated the T-LGL clone via IL-6 and activated innate cells in the reported case. The increased IFNγ production in T cells following in vitro mRNA vaccine stimulation suggests that T cells may be activated either directly or via IL-6. Whether high IFNγ secretion contributed to the overactivation of innate immunity or neutropenia in vivo cannot be concluded based on the in vitro data and a single case.
REFERENCE
Hirsiger JR, Tzankov A, Alborelli I, Recher M, Daikeler T, Parmentier S, Berger CT. Case Report: mRNA vaccination-mediated STAT3 overactivation with agranulocytosis and clonal T-LGL expansion. Front Immunol. 2023 Feb 2;14:1087502. doi: 10.3389/fimmu.2023.1087502. PMID: 36817454; PMCID: PMC9933345.


Comments
Post a Comment